
In nature, small amounts of carbon disulfideĪre found in gases released to the earth's surface as, forĮxample, in volcanic eruptions or over marshes. Carbon disulfide (CS 2) has been historically associated with the production of rayon, cellophane, and carbon tetrachloride. An interesting case is the content of Carbon Disulfide (CS2) in the petrochemical naphtha. Neurologic effects, including behavioral and. Nausea, vomiting, dizziness, fatigue, headache, mood changes, lethargy, blurred vision, delirium, and convulsions have also been reported in humans acutely exposed by inhalation. It easilyĮxplodes in air and also catches fire very easily. Acute (short-term) inhalation exposure of humans to carbon disulfide has caused changes in breathing and chest pains.

Processes is a yellowish liquid with an unpleasant odor, likeĬarbon disulfide evaporates at room temperature,Īnd the vapor is more than twice as heavy as air. The impure carbon disulfide that is usually used in most industrial Liquid with a pleasant odor that is like the smell of chloroform. List sites identified by the Environmental Protection Has been found in at least 210 of the 1,430 National Priorities Sleeping, and slight changes in the nerves. Carbon disulfide easily forms explosive mixtures with air and catches fire very easily it is dangerous when. Long periods may result in headaches, tiredness, trouble Very highly flammable, very low flash point. Very high levels can be life threatening because of itsĮffects on the nervous system. How you are exposed, personal traits and habits, and whether other chemicals are present.ĭisulfide can occur by breathing it in the air and byĭrinking water or eating foods that contain it. The effects of exposure to any hazardous substance depend on the dose, the duration, This information is important because this substance may harm you.
CARBON DISULFIDE CS2 SERIES
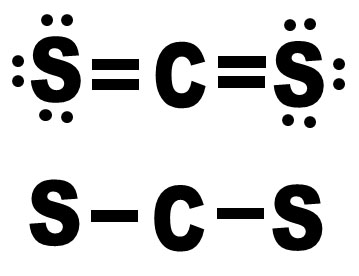
This fact sheet is one in a series of summaries about hazardous substances and their health effects. For more information, you may call the ATSDR Information Center at 1-88. Element: Carbon Symbol: C Atomic Mass: 12.0107 of Atoms: 1 Mass Percent: 15.774 Element: Sulfur Symbol: S Atomic Mass: 32.065 of Atoms: 2 Mass Percent: 84.226 Similar chemical formulas Note that all formulas are case-sensitive. This fact sheet answers the most frequently asked health questions about carbon disulfide. Has the federal government made recommendations to protect human health?.Molar Mass (g/mol) 76.143 ° (kJ/mol) So 1/ (mol-K) 237.8 AG°f kJ/mol) 115.3 65.1 CS2 (g) CS2 (g) 76.143 87.9 151.0 63.6 2nd attempt W See. Carbon disulfide (CS2) is a chemical intermediate best known for its historical use in the production of rayon, cellophane, and carbon tetrachloride. Is there a medical test to show whether I've been exposed to carbon disulfide? 05 Question (1 point) Using standard the thermodynamic properties shown below estimate the normal boiling point of carbon disulfide (CS2).How likely is carbon disulfide to cause cancer? Carbon disulfide Write a review anhydrous, 99 Empirical Formula (Hill Notation): CS2 CAS Number: 75-15-0 Molecular Weight: 76.How can carbon disulfide affect my health?.How might I be exposed to carbon disulfide?.What happens to carbon disulfide when it enters the environment?.These are just some of the reasons many industrial and clinical labs, as well as technical inspectors give us their samples for parameter analysis. Its thermal decomposition products include. We will do everything we can to find the best solution.Ī team composed of scientists will follow every stage of your project. Aim: The most important industrial use of carbon disulfide (CS2) has been in the fabrication of regenerated cellulose rayon by the viscose process and. Irradiation of gaseous carbon disulfide CS2(g) at 313 nanometers produces a dark brown aerosol of (CS2). If you have specific requirements, please let us know when requesting your tests. We choose them carefully according to your specifications et based on the techniques and analysis methods they use. Most of our laboratories are certified and/or accredited. Our objective first and foremost is to connect you with laboratories we partner with providing the analysis you need. Through optimizing the structure of reactants, intermediates, transition states, and products, it can be seen that the first step of CS2 is that CS2 reacts with H2O first to form COS intermediate and the second step is COS intermediate reacts with H2O to form H2S and CO2. For this analysis, the lab adopts the following method: Inhouse. Density functional theory (DFT) is used to look into the two-step hydrolysis mechanism of CS2. The decomposition of carbon disulfide, CS2, to carbon monosulfide, CS, and sulfur is first order with k 2.8 x 10-7 s-1 at 1000oC. Specimens must be collected using specialist equipment. Template:Chembox new Carbon disulfide is a colorless, volatile liquid with the formula CS 2. The parameter : Carbon disulfide (CS2), n☇5-15-0 is measured by HS/GC-MS.


 0 kommentar(er)
0 kommentar(er)
